
INVESTOR EVENT
06 SEPTEMBER 2022
Rare Disease
ALBIE LIVING WITH LAL-D

In order, among other things, to utilise the 'safe harbour' provisions of the US Private Securities Litigation Reform Act of 1995, AstraZeneca (hereafter ‘the Group’) provides the
following cautionary statement: this document contains certain forward-looking statements with respect to the operations, performance and financial condition of the Group,
including, among other things, statements about expected revenues, margins, earnings per share or other financial or other measures. Although the Group believes its expectations are
based on reasonable assumptions, any forward-looking statements, by their very nature, involve risks and uncertainties and may be influenced by factors that could cause actual
outcomes and results to be materially different from those predicted. The forward-looking statements reflect knowledge and information available at the date of preparation of this
document and the Group undertakes no obligation to update these forward-looking statements. The Group identifies the forward-looking statements by using the words 'anticipates',
'believes', 'expects', 'intends' and similar expressions in such statements. Important factors that could cause actual results to differ materially from those contained in forward-looking
statements, certain of which are beyond the Group’s control, include, among other things: the risk of failure or delay in delivery of pipeline or launch of new medicines; the risk of
failure to meet regulatory or ethical requirements for medicine development or approval; the risk of failure to obtain, defend and enforce effective IP protection and IP challenges by
third parties; the impact of competitive pressures including expiry or loss of IP rights, and generic competition; the impact of price controls and reductions; the impact of economic,
regulatory and political pressures; the impact of uncertainty and volatility in relation to the UK’s exit from the EU; the risk of failures or delays in the quality or execution of the Group’s
commercial strategies; the risk of failure to maintain supply of compliant, quality medicines; the risk of illegal trade in the Group’s medicines; the impact of reliance on third-party
goods and services; the risk of failure in information technology, data protection or cybercrime; the risk of failure of critical processes; any expected gains from productivity initiatives
are uncertain; the risk of failure to attract, develop, engage and retain a diverse, talented and capable workforce; the risk of failure to adhere to applicable laws, rules and regulations;
the risk of the safety and efficacy of marketed medicines being questioned; the risk of adverse outcome of litigation and/or governmental investigations; the risk of failure to adhere to
increasingly stringent anti-bribery and anti-corruption legislation; the risk of failure to achieve strategic plans or meet targets or expectations; the risk of failure in financial control or
the occurrence of fraud; the risk of unexpected deterioration in the Group’s financial position; and the impact that global and/or geopolitical events such as the COVID-19 pandemic
and the Russia-Ukraine war, may have or continue to have on these risks, on the Group’s ability to continue to mitigate these risks, and on the Group’s operations, financial results or
financial condition. Nothing in this document, or any related presentation/webcast, should be construed as a profit forecast.
2
Forward-looking statements

Rare Disease Investor Event | Agenda
3
I. Introduction & Alexion Strategy
II. Sustained Leadership in Complement
III. Expanding Beyond Complement
IV. Geographic Expansion
V. Building Scientific Bridges
VI. Closing Remarks
VII. Q&A Session
Marc Dunoyer
Chief Executive Officer,
Alexion
Scott Weintraub
VP, Global Marketing &
Commercial Strategy
Gianluca Pirozzi
SVP, Head of
Development & Safety
Sharon Barr
SVP, Head of Research
& Product Development

4
Strategy
INTRODUCTION
AND
5
Alexion & AstraZeneca
Unique opportunity to enhance long-term value, meeting AstraZeneca strategic criteria
Aligned with
AstraZeneca
strategy
Accelerate
innovative science
AstraZeneca will be
able to add value
Potential for geographic
expansion
Supports
AstraZeneca
financial profile
Supports top-line
growth, earnings
accretive
Feasible
integration
Shared cultural
values
Alexion, AstraZeneca Rare Disease
6
1. Global Genes, https://globalgenes.org/rare-disease-facts/ 2. 1 in 10 people live with a rare disease in the US; estimated 400 million people globally diagnosed with a rare disease 3. Wakap et al. 2019;
Global Genes: RARE Facts 2020 4. Global Genes, https://globalgenes.org/ 2016 WRDD Fact Sheet.
50%
of rare disease
patients
are children
4
1 in 10
people live with
a rare disease
2
80%
of rare diseases
are genetic
1
4.8 years
average time to diagnosis;
40% receiving
> 1 misdiagnosis
3
OUR VISION is to transform the future of rare disease, increasing access to our medicines
globally and innovating to treat more patients, earlier, with greater precision and efficacy
Transforming the treatment of rare diseases

7
Alexion, AstraZeneca Rare Disease: strategic priorities
Sustained Leadership in
Complement
Expanding Beyond
Complement
Organic Innovation &
Collaboration with
AstraZeneca
8
Alexion, AstraZeneca Rare Disease: approved medicines
1
Five approved medicines indicated for seven rare diseases
1. Alexion medicines may not be approved in every geography across the globe.
FOR
Soliris
(eculizumab)
Ultomiris
(ravulizumab-cwvz)
Strensiq
(asfotase alfa)
Kanuma
(sebelipase alfa)
z
Koselugo
(selumetinib)
paroxysmal nocturnal
haemoglobinuria (PNH)
atypical haemolytic
uraemic syndrome (aHUS)
generalised
myasthenia gravis (gMG)
neuromyelitis optica
spectrum disorder
(NMOSD)
paroxysmal nocturnal
haemoglobinuria (PNH)
atypical haemolytic
uraemic syndrome (aHUS)
generalised
myasthenia gravis (gMG)
hypophosphatasia
(HPP)
lysosomal acid lipase
deficiency
(LAL-D)
neurofibromatosis
type 1 with plexiform
neurofibromas
(NF1-PN)
9
Alexion, AstraZeneca Rare Disease
Broad pipeline across many high unmet need, high value indications
1. Renal basket trial including proliferative lupus nephritis or Immunoglobulin A Nephropathy; IV = intravenous; SC = subcutaneous; HSCT-TMA = haematopoietic stem cell transplant-associated thrombotic microangiopathy;
CM-TMA = complement-mediated thrombotic microangiopathy; DM = dermatomyositis; GBS = Guillain-Barré syndrome; gMG = generalised myasthenia gravis; PNH = paroxysmal nocturnal haemoglobinuria; PNH-EVH =
paroxysmal nocturnal haemoglobinuria with extravascular haemolysis; GA = geographic atrophy; SCD = sickle cell disease; cAMR = chronic antibody-mediated rejection; NF1 = neurofibromatosis type 1; ATTR-CM =
transthyretin amyloid cardiomyopathy; AL amyloidosis = light chain amyloidosis; ng = next-generation; HPP = hypophosphatasia.
Ultomiris
(2nd generation C5)
Soliris
Complement
HSCT-TMA (IV)
Renal
1
(IV)
GBS, JP only (IV)
ALXN2040
(Factor D)
ALXN2050
(Factor D)
PNH with EVH (Oral)
PNH monotherapy (Oral)
Renal
1
(Oral)
GA (Oral)
PHASE I PHASE II PHASE III
DM (IV)
gMG (Oral)
ALXN1820 (Factor P)
SCD (SC)
Beyond
Complement
ALXN1840
CAEL-101
ALXN2060
AL amyloidosis (IV)
Wilson Disease (Oral)
ATTR-CM, Japan only (Oral)
ALXN1850 (ngHPP) HPP (SC)
ALXN1910 NF1 (SC)
NI006 (TTR depleter) ATTR-CM (IV)
Koselugo NF1 adult (Oral)
ALXN2030
cAMR (SC)
ALXN2080 (Factor D)
Oral
CM-TMA (IV)
ALXN1720 (3rd generation C5)
gMG (SC)

10
CHELSEY LIVING WITH NMOSD
Complement
SUSTAINED
LEADERSHIP IN
Broad expertise in complement biology
11
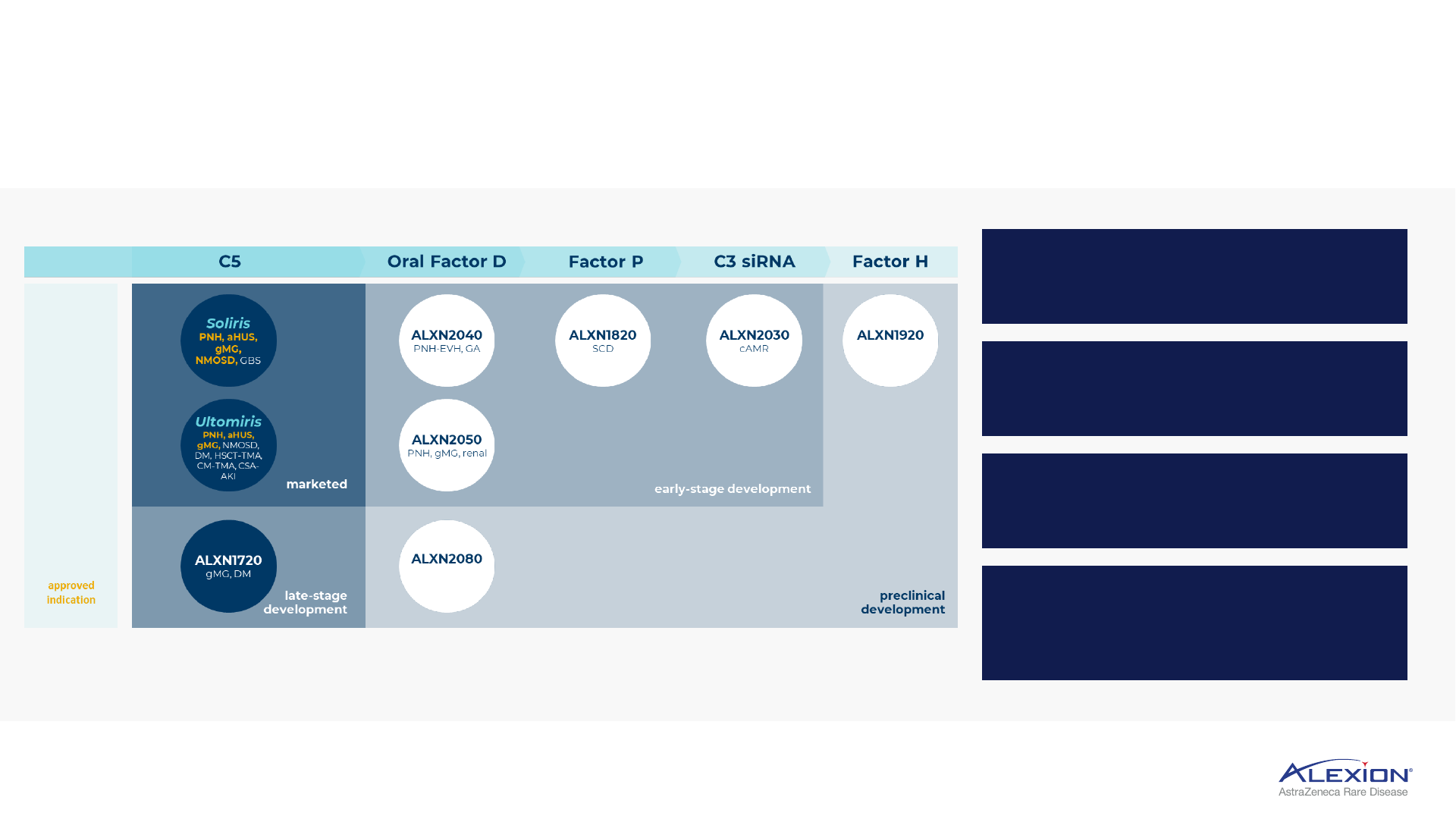
Multiple development-stage platforms, leveraging foundational complement expertise
Soliris
PNH, aHUS,
gMG,
NMOSD, GBS
ALXN1720
gMG, DM
Ultomiris
PNH, aHUS,
gMG, NMOSD,
DM, HSCT-TMA,
CM-TMA, CSA-
AKI
ALXN2040
PNH-EVH, GA
ALXN2080
ALXN2050
PNH, gMG, renal
ALXN1820
SCD
ALXN2030
cAMR
ALXN1920
C5 Oral Factor D
Factor P
C3 siRNA Factor H
approved
indication
late-stage
development
marketed
early-stage development
preclinical
development
PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic syndrome; gMG = generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder; GBS = Guillain-Barré syndrome; DM =
dermatomyositis; HSCT-TMA = haematopoietic stem cell transplant-associated thrombotic microangiopathy; CM-TMA = complement-mediated thrombotic microangiopathy; CSA-AKI = cardiac surgery-associated acute
kidney injury; PNH-EVH = paroxysmal nocturnal haemoglobinuria with extravascular haemolysis; GA = geographic atrophy; SCD = sickle cell disease; siRNA = small interfering RNA; cAMR = chronic antibody-mediated
rejection.
12
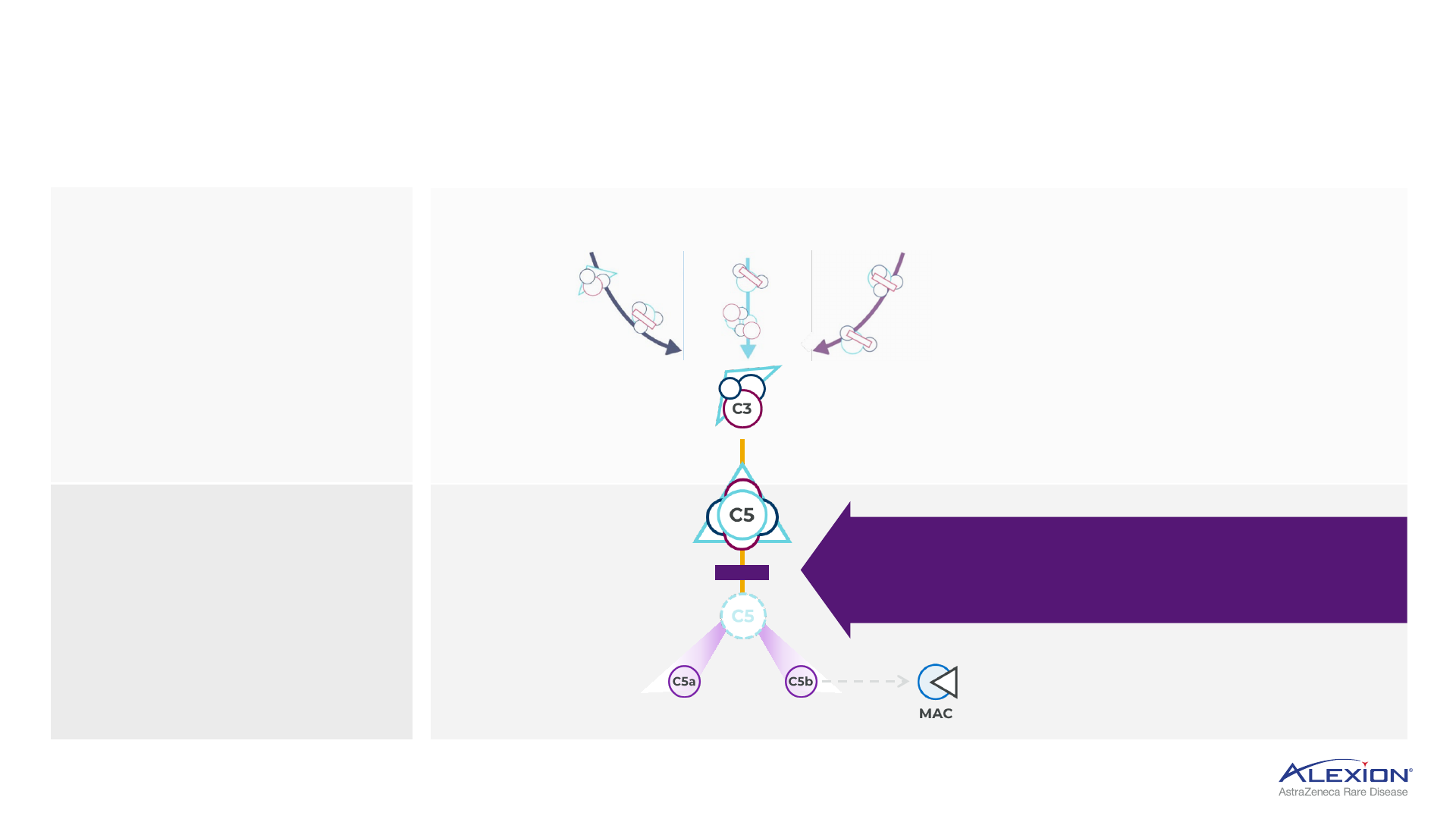
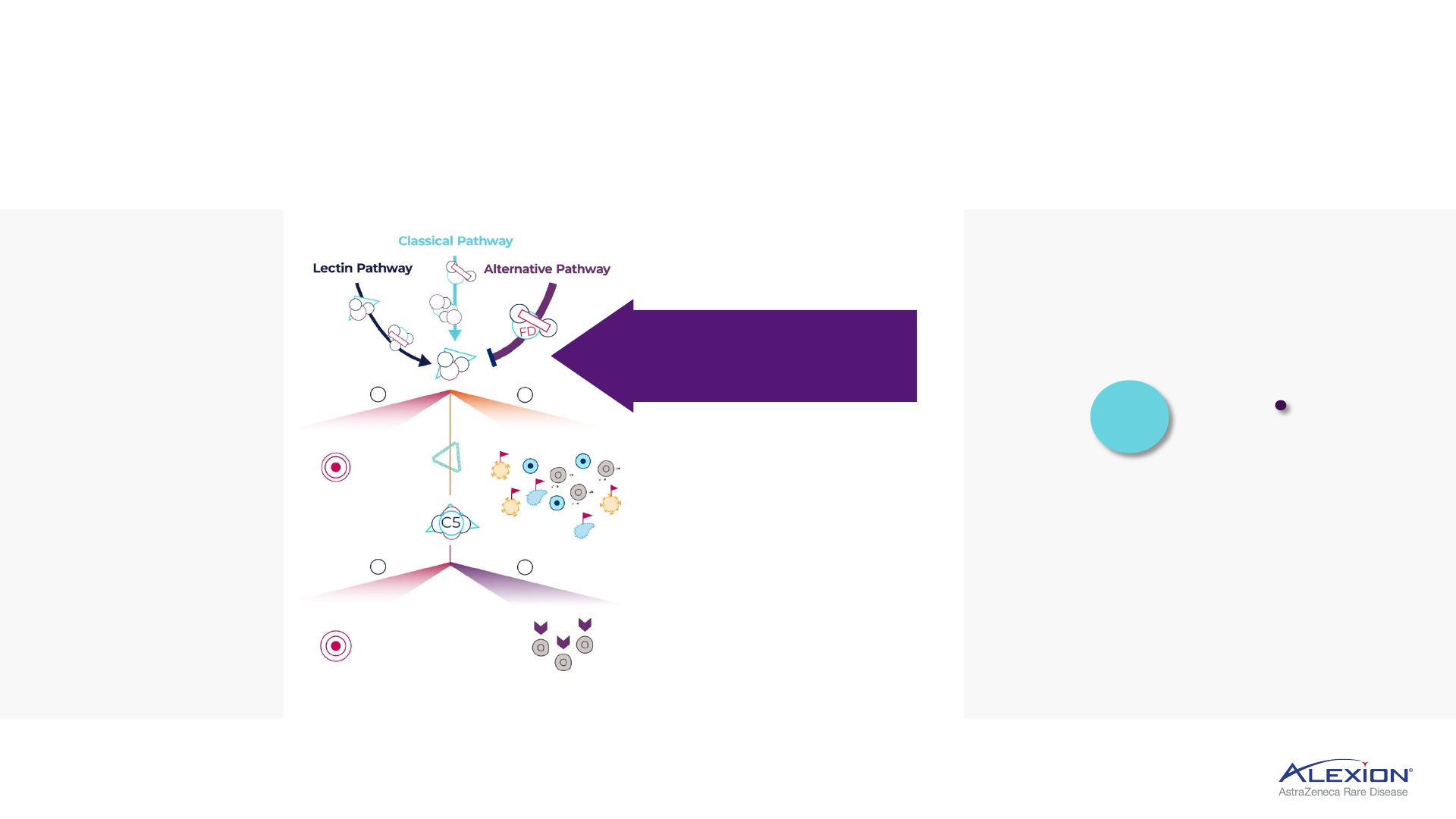
Foundation in terminal complement (C5) inhibition
Several areas of complement cascade are implicated in disease pathology
Lectin
Pathway
Classical
Pathway
Alternative
Pathway
Inhibiting terminal complement prevents
unwanted cell destruction and inflammation,
leaving proximal complement pathways intact
C5 protein splits to
form C5a and C5b
C5a triggers
inflammation
C5b triggers assembly of C6 to C9 into
MAC, leads to cell destruction
Complement system consists
of >30 proteins activated
in a cascade to maintain
homeostasis in the body
Complement activation
results in formation of MAC,
which leads to destruction
of target cells
Overactivation of
complement can trigger
uncontrolled cascade
of reactions that
damage tissues
C3 broken down into C3a and C3b
and binds to C3 convertase to form
C5 convertase
C6-9
MAC = membrane attack complex.

13
Foundation in terminal complement (C5) inhibition
Diverse C5 inhibitor portfolio, optimised for differentiated indication selection
Q2W = every 2 weeks; IV = intravenous; Q8W = every 8 weeks; QW = once-weekly; SC = subcutaneous; V
H
H Ab = single domain antibody; PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic
syndrome; gMG = generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder; HSCT-TMA = haematopoietic stem cell transplant-associated thrombotic microangiopathy; CSA-AKI = cardiac surgery-
associated acute kidney injury; DM = dermatomyositis.
ALXN1720
C5 bi-specific mini-body (V
H
H Ab)
Q2W IV Q8W IV, QW SC QW SC
PNH
gMG
aHUS
NMOSD
CSA-AKI
HSCT-TMA
gMG DM
DM
PNH
gMG
aHUS
NMOSD
Continued innovation, expanding into broader patient populations

Establishing Ultomiris as the new standard of care
14
Value proposition supports rapid facilitated conversion and growth
1. Depicted annual treatment cost differentials calculated based on US list prices. 2. Ultomiris NMOSD regulatory decision anticipated in H1 2023 in US and EU. 3. Defined as >70% conversion in the US. 4. US addressable
population estimated to be 30k, representing a 3-fold increase compared to Soliris addressable population (8-10k). PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic syndrome; gMG =
generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder; Q8W = every 8 weeks.
Ultomiris vs. Soliris pricing dynamics
1
+10%
-18%
-10%
-33%
Lower average annual treatment cost per patient
PNH
aHUS, gMG, NMOSD
2
PNH
best-in-class conversion,
reaching saturation in
key markets
aHUS
market share leader,
variable duration of
treatment
gMG
c.30k addressable
population (3x Soliris)
4
,
including naïve and
switch
NMOSD
best-in-class efficacy,
Q8W dosing expands
addressable patient
population; potential
approval H1 2023
Ultomiris potential to achieve best-in-class
conversion across four Soliris-labelled indications
by 2025
3
YEAR 1
YEAR 2+
Loading dose + maintenance dosing
Maintenance dosing
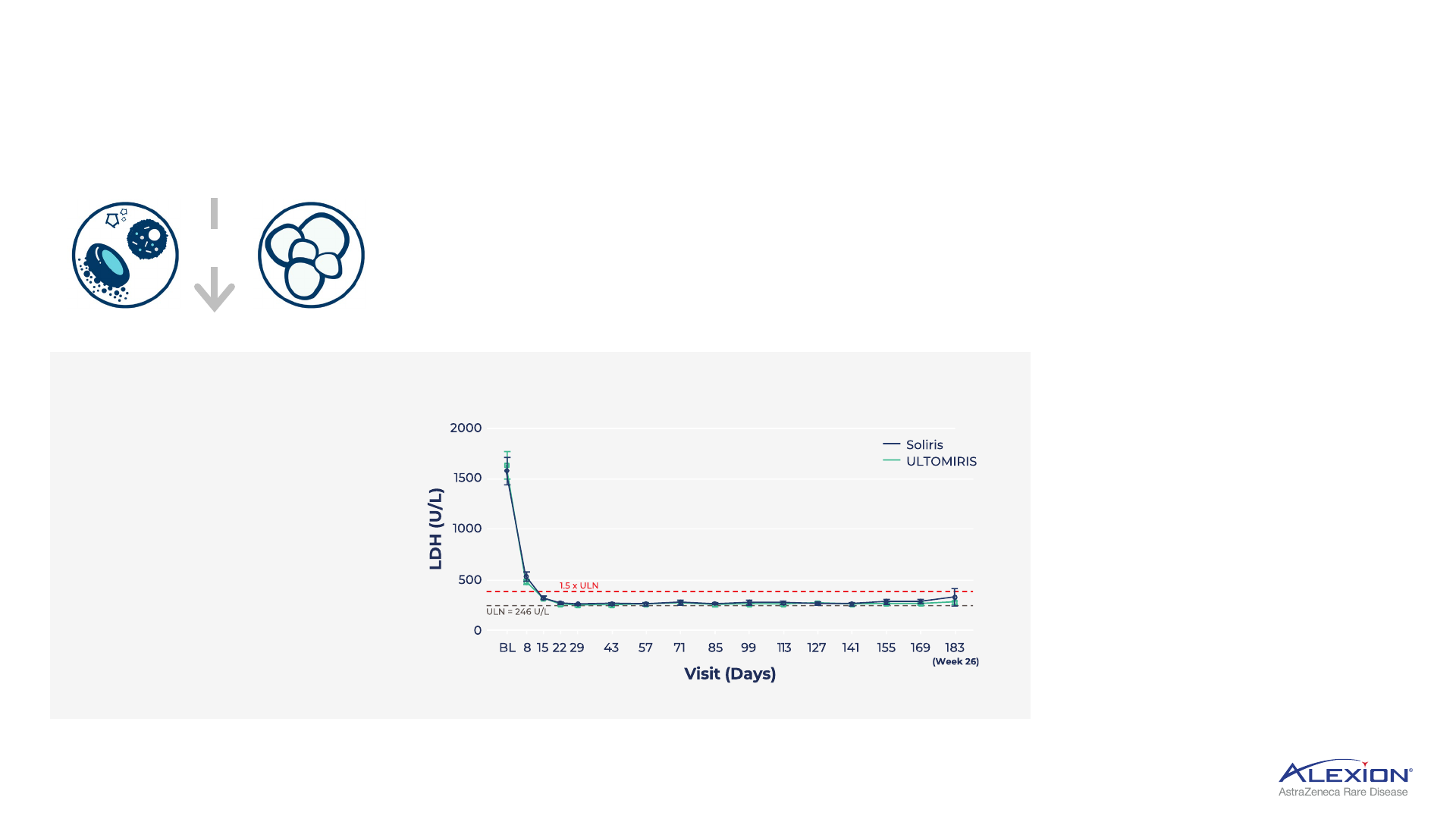
Compelling, durable C5 data in PNH
15
Pivotal 301 trial and longest registry data to date solidifies Ultomiris as standard of care
1. Kulasekararaj, Brodskey, Griffin et al., “Long-term complement inhibition and survival outcomes in patients with paroxysmal nocturnal haemoglobinuria: an interim analysis of the ravulizumab clinical trials.” 2.
Hillmen et al., 1995. 3. Lee et al., 2013; Jang et al., 2016 4. Schrezenmeier et al., “Predictors for Improvement in Patient-Reported Outcomes: Post-Hoc Analysis of a Phase III Randomised, Open-Label Study of Eculizumab
and Ravulizumab in Complement Inhibitor-Naïve Patients with Paroxysmal Nocturnal Haemoglobinuria (PNH).” PNH = paroxysmal nocturnal haemoglobinuria; IVH = intravascular haemolysis; LDH = lactate
dehydrogenase; ULN = upper limit of normal.
6-year survival analysis
of >450 patients with
PNH Ultomiris
1
97.5%
~65%
Historical 5-year
survival rates in PNH
patients with evidence
of haemolysis not on
anti-C5 treatment
2
SURVIVAL RATES REPORTED
IN PNH REGISTRY TRIAL
In PNH, uncontrolled terminal
complement activity leads to IVH;
LDH is key biomarker of IVH
• Ultomiris demonstrated
rapid and sustained reductions
in LDH, with mean levels
remaining stable and < 1.5 × ULN
2
• LDH levels >1.5 × ULN is predictor
for risk of thrombosis and
mortality in PNH
3
• LDH is one of the strongest
predictors for improvement
in patient-reported clinical
outcomes
4
MEAN LDH LEVELS OVER TIME IN STUDY 301
+
IVH
Thrombosis
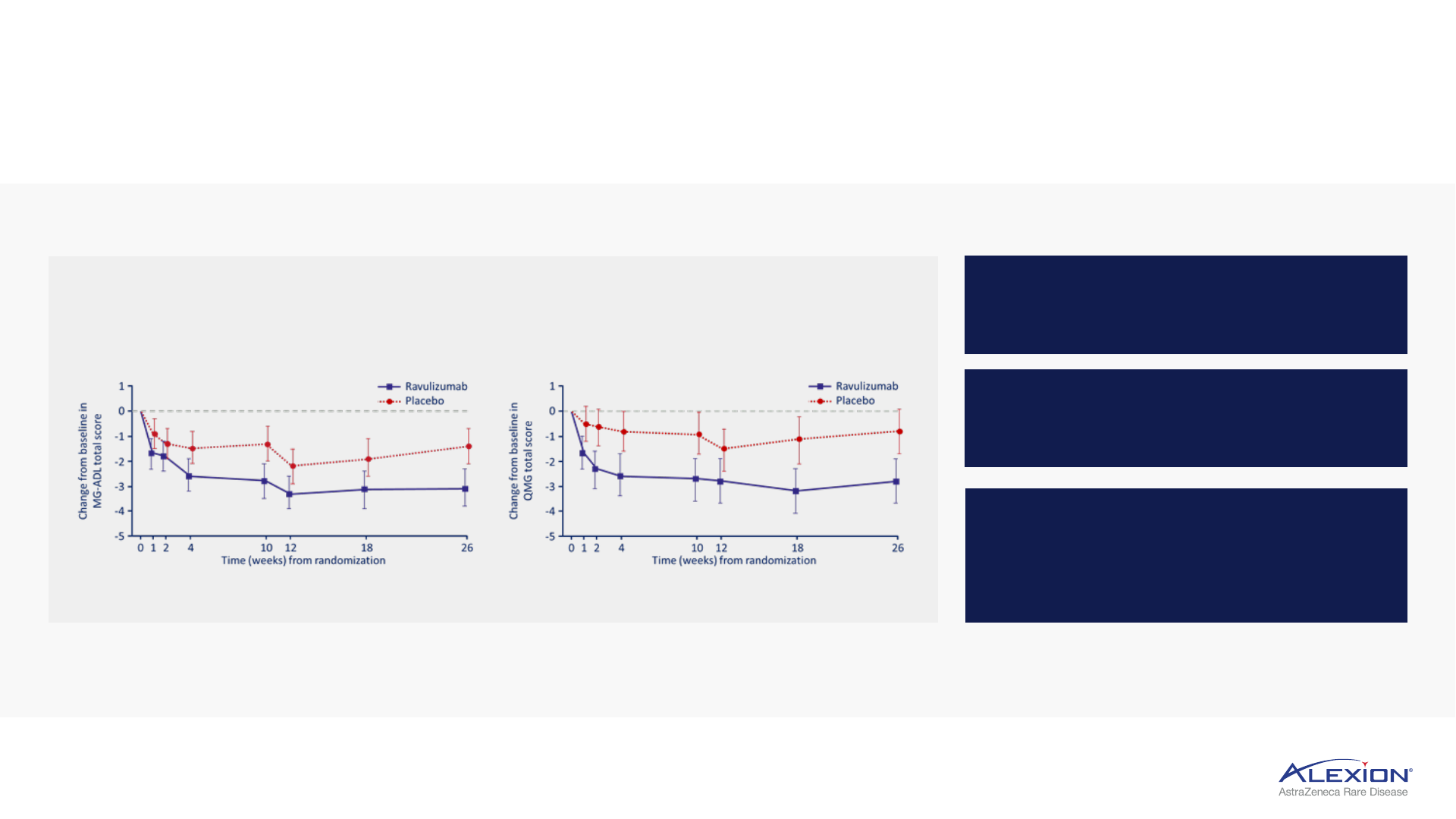
Ultomiris Phase III HLR confirms C5 leadership in gMG
16
Rapid and sustained improvement in key gMG measures of clinical benefit
1. Myasthenia Gravis Activities of Daily Living is an 8-item outcome measure that reflects ocular, bulbar, respiratory and limb symptoms and their impact on function. 2. Quantitative Myasthenia Gravis scale is a 13-item
evaluation of ocular, facial, bulbar, gross motor, axial and respiratory weakness. 3. Vu, Meisel, Mantegazza, et al. presented at Muscular Dystrophy Association Conference 2020; 2020, virtual. HLR = high-level results;
gMG = generalised myasthenia gravis; MG-ADL = myasthenia gravis activities of daily living; QMG = quantitative myasthenia gravis.
Significant improvement in patient (MG-ADL)
1
and
physician-reported (QMG)
2
assessments
3
Improvement observed in one week
on MG-ADL and QMG measures
Improvements sustained through
the 60-week follow-up period
76% of patients in the Ultomiris arm
experienced clinically meaningful
improvement on MG-ADL (49% on
QMG) by week 60
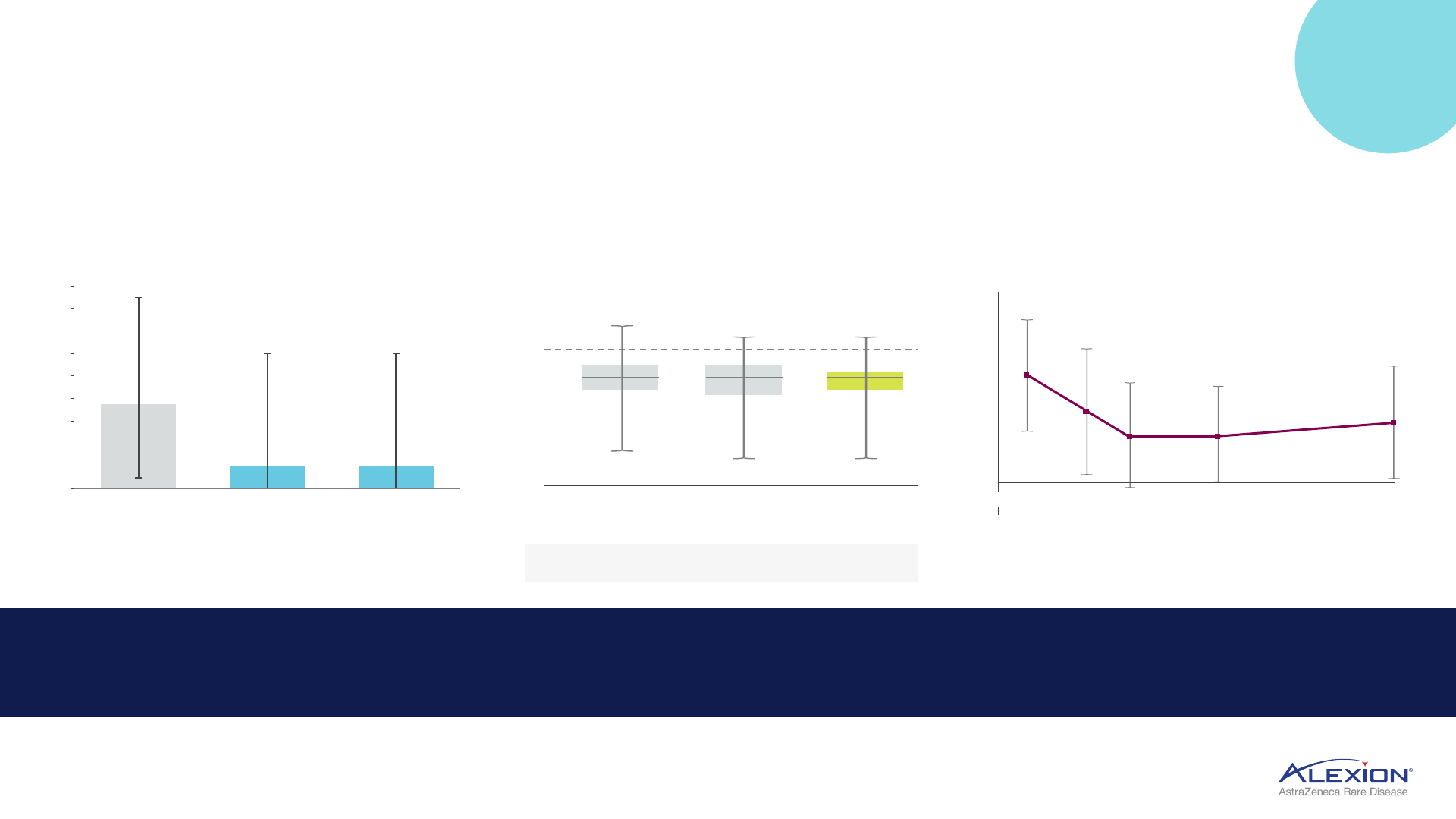
+
+ +
Durable C5 efficacy in gMG
gMG registry shows 5.5-point MG-
ADL reduction with treatment
Japan PMS trial shows 5-point MG-
ADL reduction at 52 weeks
Project ELEVATE trial resulted in
>3-point MG-ADL reduction at 24 months
Soliris global registry trials reinforce benefit of long-term, continuous treatment
7.5
2.0
2.0
0
2
4
6
8
10
12
14
16
18
Before
treatment
During treatment
(at enrolment - 1.8y
after initiation)
During treatment
(at last follow-up -
2.8y after initiation)
n=28
Median MG-ADL
n=136
MG-ADL total score
Mean (SD)
change
-4.11 -4.88 -5.00
(3.49) (3.93) (4.15)
Change from baseline in MG-ADL score
Time from Soliris initiation (week)
Time after
Soliris initiation (months)
Mean (SD) MG-ADL total score over time
Before
Soliris
initiation
8 -
4 -
0 -
-4 -
-8 -
-12 -
-16 -
-20 -
12 (N=93) 26 (N=90) 52 (N=46)
-3 0 3 6 9 12 15 18 21 24
14 -
12 -
10 -
8 -
6 -
4 -
2 -
0 -
8.0
84
5.4*
51
57
3.7* 3.8*
58 37
4.7*
17
NEW
DATA
46.4% of patients reached MSE
status during treatment
Only 26.4% of patients on
corticosteroids (≤5 mg/day) at 52 wks
72% of patients reduced or
discontinued steroids
1. Muppidi et al. presented at International Congress on Neuromuscular Diseases 2022; July 2022, Brussels. 2. Murai et al, "Clin Exp Neuroimm;" May 2022. 3. Habib et al. presented at American Academy of Neurology
2022; April 2022, Seattle. gMG = generalised myasthenia gravis; MG-ADL = myasthenia gravis activities of daily living; MSE = minimal symptom expression; y = year; PMS = post-marketing surveillance; SD = standard
deviation; wks = weeks.
18
Ultomiris Phase III HLR confirms C5 leadership in NMOSD
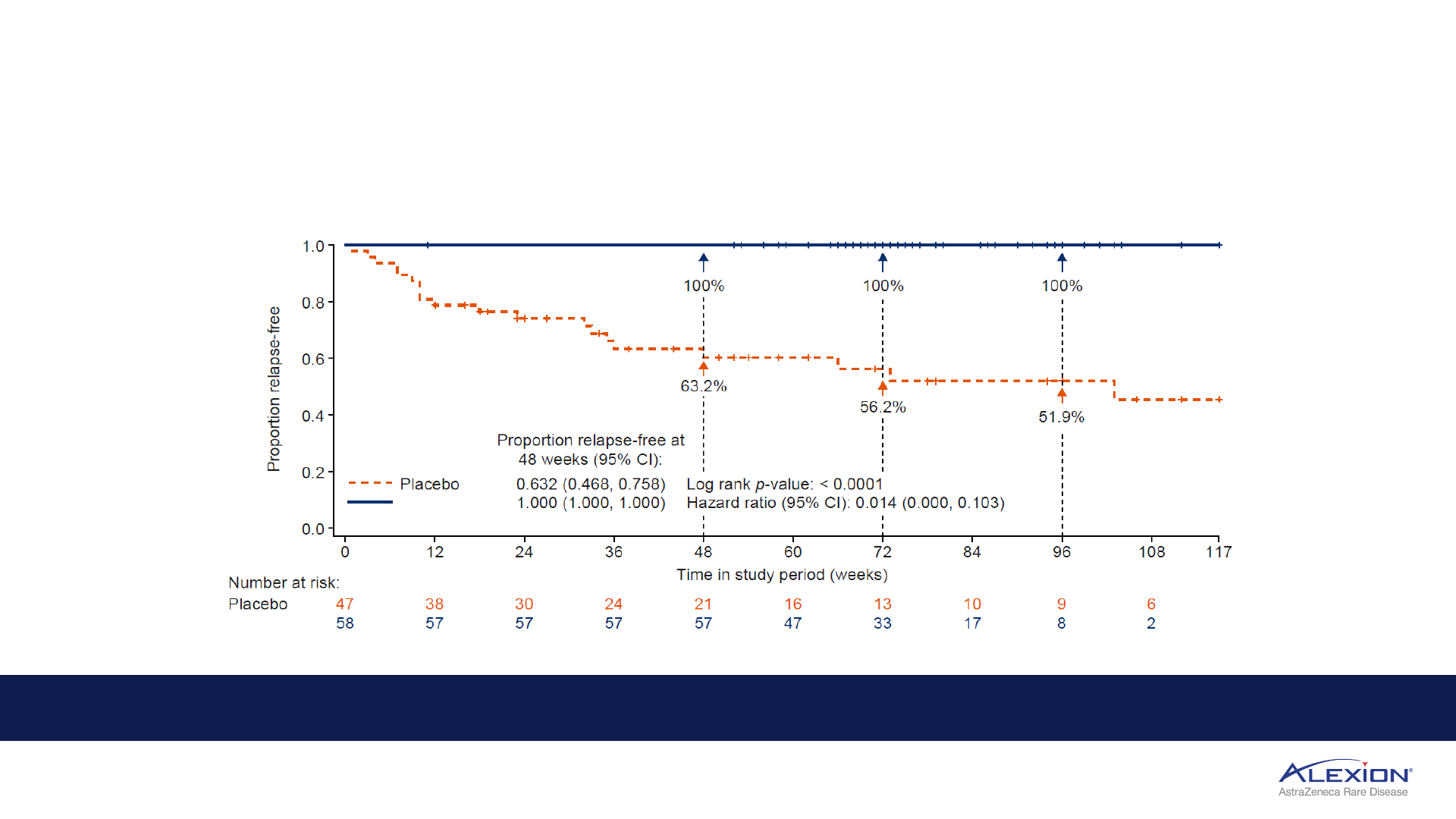
Anticipated regulatory decision in H1 2023 (US, EU)
1. Friedemann, Pittock, Barnett, Bennett, Berthele, et al. presented at European Academy of Neurology 2022; June 2022, Vienna. 98.6% reflects model adjusted risk of relapse. HRL = high-level results; NMOSD =
neuromyelitis optica spectrum disorder; EU = European Union; CI = confidence interval.
Ultomiris reduced the risk of relapse by 98.6% compared with placebo in CHAMPION-NMOSD trial
1
Ultomiris
Ultomiris
Zero adjudicated relapses in Ultomiris arm over 73.5-week median treatment period

Ultomiris indication expansion will continue
19
Direct-to-Phase III trials and potential blockbuster opportunities in HSCT-TMA, CSA-AKI
1. Jodele, Dandoy, Lane, et al., “Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab.” “Blood.” HSCT-TMA = haematopoietic stem cell transplant-associated
thrombotic microangiopathy; 2. Pickering JW, James MT, Palmer SC, “Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies, Am J Kidney Dis. 2015 Feb; 2. Epidemiology
data on file; CSA-AKI = cardiac surgery associated-acute kidney injury; TMA = thrombotic microangiopathy; EU5 = France, Germany, Italy, Spain, United Kingdom; Top 8 = US, EU5, JP, CN; AKI = acute kidney injury; CKD
= chronic kidney disease; CPB = cardiopulmonary bypass.
CSA-AKI
30k
US
Patients with
CKD at risk of
AKI following
CPB
2
Single-dose Ultomiris, pre-surgery has potential to
prevent CSA-AKI
60-80%
of mod-to-severe
CKD patients experience
AKI post-CPB
2
patients
develop AKI
post-surgery
2
1/4
HSCT-TMA
c.80% rate of mortality with no approved medicines
c.9k diagnosed
patients in US,
EU5, JP
2
Proof-of-concept
established with
Soliris in HSCT-TMA
1
Significantly improved
survival outcomes
vs. historical controls
AKI post-CPB
can result in increased
hospital mortality
2
17k
EU5
7k
Japan
Ultomiris potential to be first-and-only medicine for HSCT-TMA, and first-and-only preventative therapy for CSA-AKI
Ultomiris geographic expansion
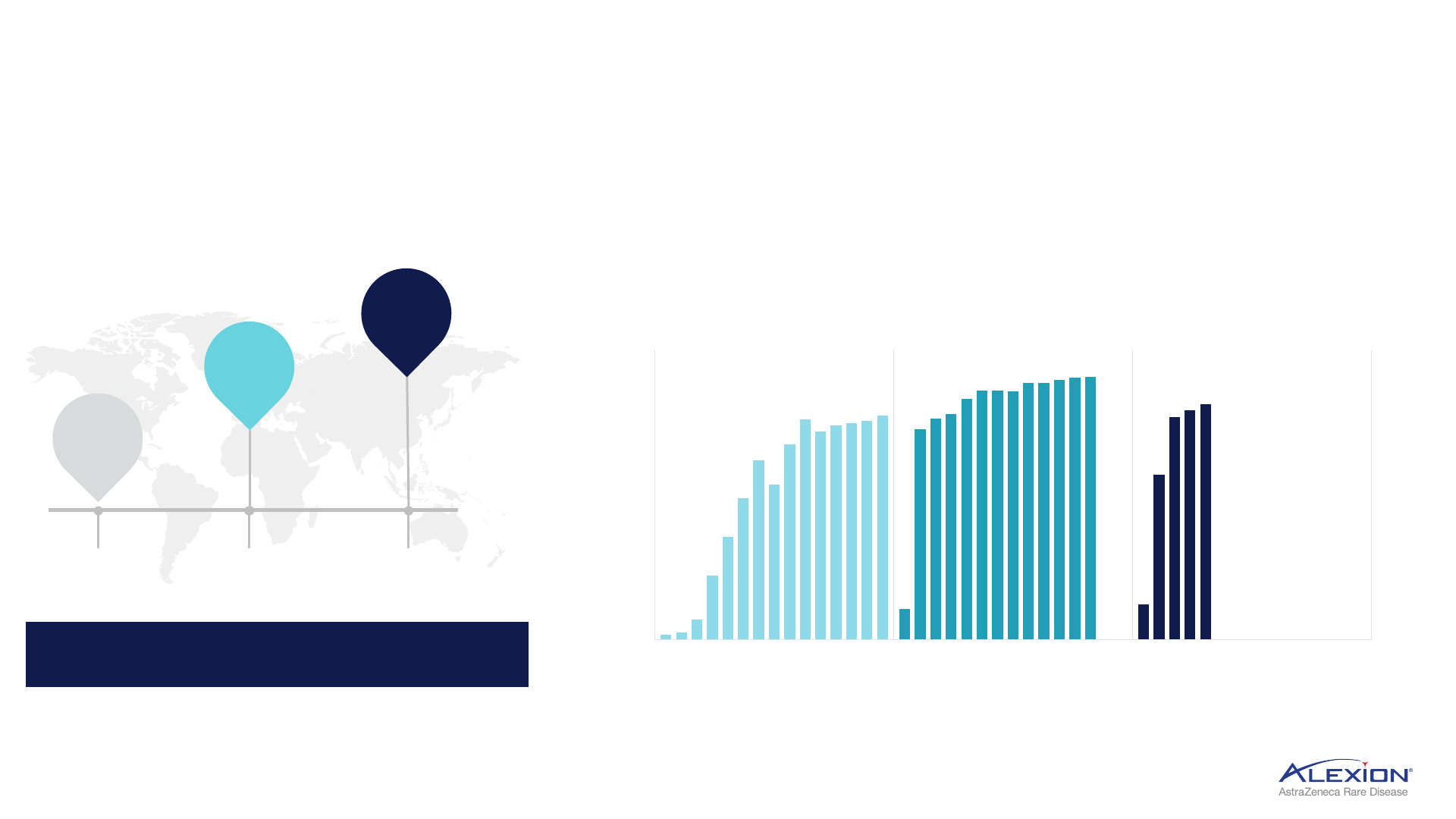
20
Significant market expansion underway, demonstrated rapid conversion upon launch
1. Represents total FDA, MAA submissions, total count does not include additional indication submissions; cumulative filing count 2. Ultomiris patient share average calculated based on data from Germany, UK, Italy,
France and Australia. PNH = paroxysmal nocturnal haemoglobinuria; FDA = Food and Drug Administration; MAA = Marketing Authorisation Application; UK = United Kingdom.
Accelerating pace of Ultomiris
launches
1
globally
58
65
85
Ultomiris in 85 countries by 2023
Rapid PNH conversion to Ultomiris in new country launches
c.80% of PNH patients convert to Ultomiris within 12 months of launch
2
2022
2023
2021
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Country A Country B
Country C
months from launch
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 1 2 3 4 5 6 7 8 9 10 11 12 13 1 2 3 4 5
Conversion case study: PNH conversion in three recent country launches
21
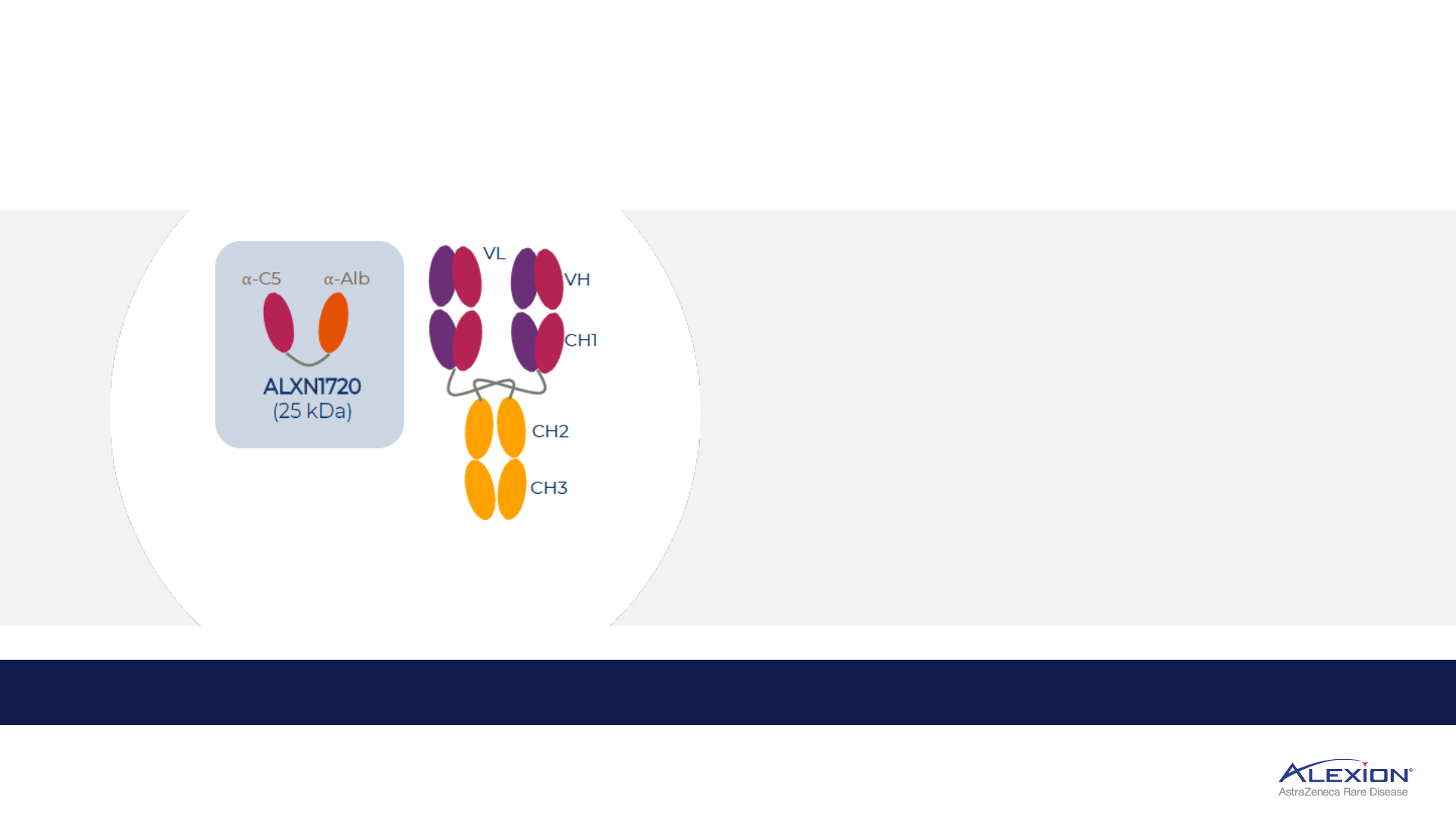
ALXN1720 supports further indication expansion
Third-generation C5 mini-body (V
H
H Ab), potential best-in-class SC administration
1. Direct to Phase III strategy. V
H
H Ab = single domain antibody; SC = subcutaneous; ; QW = once-weekly; self-admin = self-administered; gMG = generalised myasthenia gravis; YE = year-end; DM = dermatomyositis.
• Low molecular weight, QW self-admin auto-injector
• Differentiated pricing expands indication opportunities
• Potential best-in-class SC in gMG with potential to capture
majority of self-admin market share
Soliris
(150 kDa)
ALXN1720 demonstrated strong safety and tolerability
profile in Phase I, initiating Phase III in gMG by YE2022
ALXN1720, QW low-volume SC, supporting neurology expansion in gMG
1
and DM

C5 inhibition in Dermatomyositis
22
Potentially first novel mechanism, PoC complement inhibition data underway
PoC = proof-of-concept; MAC = membrane attack complex; SoC = standard of care; OLE = open-label extension; Top 8 = US, EU5, JP, CN; EU5 = France, Germany, Italy, Spain, United Kingdom.
Autoimmune inflammatory
myopathy
Established role of
complement
Establishing PoC with Ultomiris
Evidence of
MAC deposition
in transverse
vessel, leading to
destruction of
muscle fiber
• Inflammation
causing painful,
itchy skin rashes
across body
• Progressive
muscular
weakness may
lead to respiratory
failure and death
Ultomiris Phase II/III PoC underway,
with potential to pursue Ultomiris and
ALXN1720 for commercialisation
randomised control
period: 26 weeks
R 2:1
Ultomiris
OLE
Ultomiris + SoC
placebo + SoC
Part A
(n=48)
randomised control
period: 50 weeks
R 1:1
Ultomiris
Ultomiris + SoC
placebo + SoC
Part B
(n=132)
OLE
Significant unmet need, limited competition with c.189k diagnosed patients in Top 8 countries
Expanding into proximal complement (AP) inhibition
23
Novel small molecule, oral Factor D portfolio with ALXN2040, ALXN2050, ALXN2080
1. Based on human protein concentrations in the plasma; AP = alternative pathway.
Factor D more likely to
maintain consistent control
than Factor B inhibitors
2.15
μM
AP only
Factor B
1
Factor D
1
AP only
0.08 μM
• Factor D more tractable target given
lower circulating concentration in plasma
• Factor B is an acute phase reactant,
circulating levels increase during
inflammation
Alternative Pathway
(AP) dysregulation
triggers uncontrolled
cascade of reactions,
which may lead to
cell destruction and
harmful inflammation
In vitro analysis of small
molecules, ALXN2040
and ALXN2050,
demonstrate high affinity
for Factor D and
significantly reduced
complement-mediated
haemolysis at low
concentrations
Factor D inhibition
blocks AP, leaving
classical and lectin intact
24
Oral Factor D portfolio
Four PoC read-outs over next 18 months, un-gating several Phase III starts
PoC = proof-of-concept; PNH-EVH = paroxysmal nocturnal haemoglobinuria with extravascular haemolysis; GA = geographic atrophy; PNH = paroxysmal nocturnal haemoglobinuria; gMG = generalised myasthenia gravis; LN
= lupus nephritis; IgAN = immunoglobulin A nephropathy.
ALXN2040
danicopan
ALXN2050
vemircopan
ALXN2080
Potential first oral medicine
in Geographic Atrophy
Positive PoC Phase II
PNH monotherapy
Potential application in
non-rare indications
Phase III PNH-EVH add-on underway Phase II PNH monotherapy, gMG and
renal (LN, IgAN) underway
Entering clinic in 2022
Ongoing Phase II trial GA monotherapy
Demonstrated PoC for Factor D inhibitors in PNH
25
Phase II ALXN2050 PoC in PNH, Phase III ALXN2040 add-on enrollment complete
PoC = proof-of-concept; PNH = paroxysmal nocturnal haemoglobinuria; EVH = extravascular haemolysis; HLR = high-level results; ULN = upper limit of normal; FDA = Food and Drug Administration; LT = long-term; IVH
= intravascular haemolysis; Hgb = haemoglobin; QOL = quality of life.
Phase III ALXN2040 trial in subset of PNH patients
with clinically significant EVH as add-on
Phase III fully enrolled, HLR H1 2023
Potential to address remaining 10-15% of PNH patients that continue
to experience clinically significant EVH
R 2:1
12 weeks
(n=~84)
12 weeks
Patients will continue their
C5 regimen throughout
study duration to ensure
continued IVH control
Ultomiris or Soliris +
ALXN2040
Ultomiris or Soliris +
placebo
Ultomiris or Soliris +
ALXN2040
ALXN2050 positive PoC in PNH monotherapy
(n=~26)
12 weeks
LT extension
1. Patients on a
C5 inhibitor with
anemia and
reticulocytes > ULN
2. PNH treatment
naïve patients
3. Patients receiving
ALXN2040
monotherapy
• Robust control of IVH and addresses EVH
• ALXN2050 resulted in 3.9 g/dL increase in Hgb
• Clinically meaningful improvements across haemolysis markers
and QoL measures at 12 weeks
Phase II data to be presented at upcoming congress
ALXN2050 ALXN2050
FDA
Breakthrough
Designation
NEW
DATA

26
Portfolio
Approach
SELECT
INDICATIONS
ROBERTA LIVING WITH gMG
27
Alexion portfolio approach in PNH
PNH market evolution requires multiple modalities to address spectrum of patient needs
PNH = paroxysmal nocturnal haemoglobinuria; EVH = extravascular haemolysis.
ALXN2040
ALXN2050
Ultomiris expected to remain standard of care for
existing and newly diagnosed PNH patients
ALXN2050 monotherapy addresses subset of PNH
patients who prefer and will be compliant on oral
Ultomiris
ALXN2040 add-on to C5 inhibitors with potential to
address c.10% of patients with clinically significant EVH
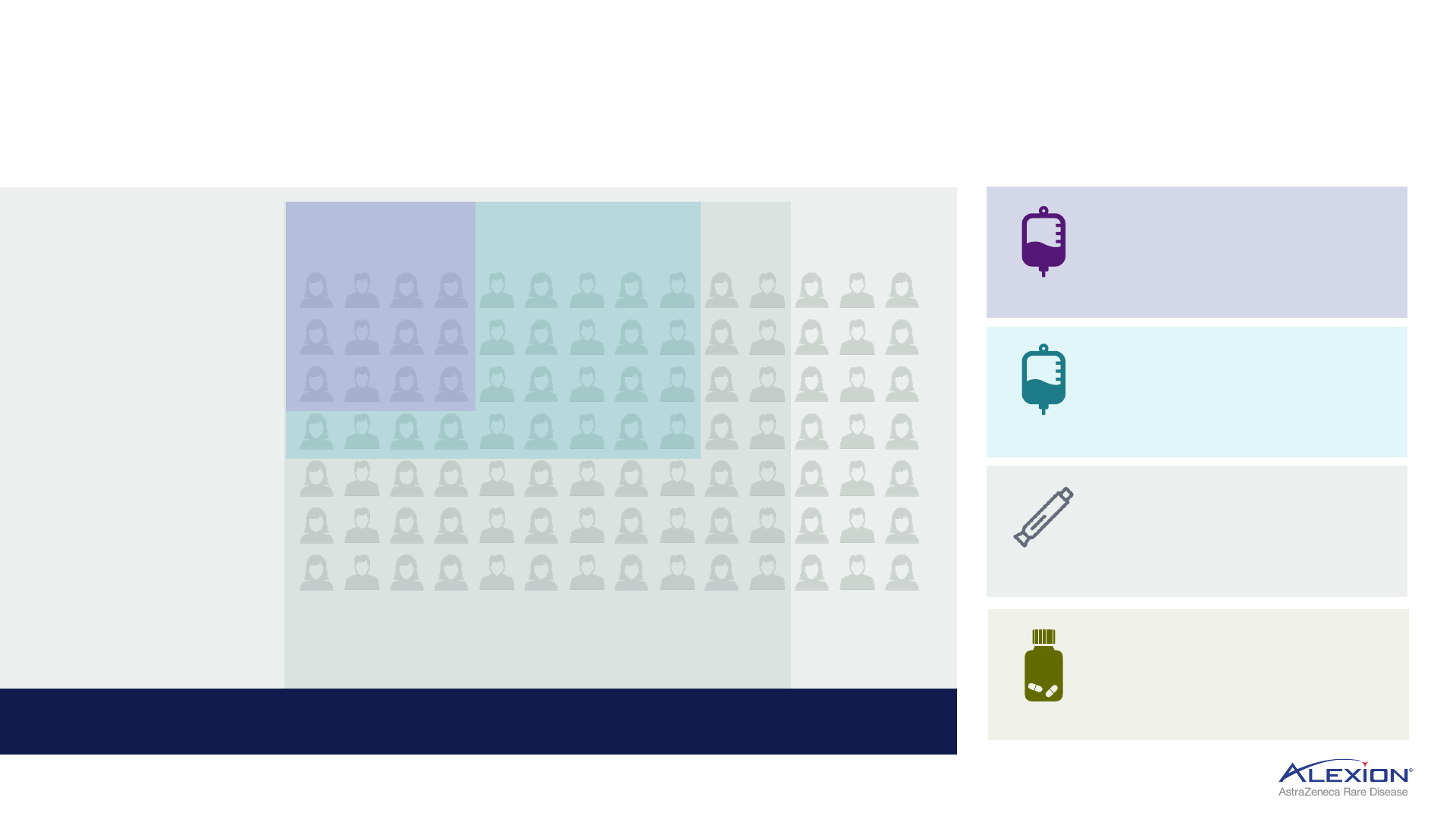
Ultomiris
c.30k
1
addressable
ALXN1720 (QW SC) and ALXN2050 (oral Factor D)
potential to expand further given consistent efficacy,
patient-friendly RoA, differentiated pricing
Soliris
refractory c.8-10k
1
Alexion portfolio approach in gMG
28
Complement inhibitors offer sustained symptom control and disease improvement
1. US gMG population. gMG = generalised myasthenia gravis; QW = once-weekly; SC = subcutaneous; RoA = route of administration; FPCD = first patient commenced dosed; Q2W = every 2 weeks; Q8W = every 8 weeks;
BID = twice-daily; SoC = standard of care; IST = immunosuppressive therapy.
Soliris
• SoC for refractory patients
• Proven foundational efficacy
of C5 inhibition in gMG
Q2W
Ultomiris
• First branded choice with
durable, sustained efficacy
• Improved dosing profile
ALXN1720
Additional, convenient dosing
option for improved patient
experience
ALXN2050
Innovative oral to break IST
cycling for less severe patients
Q8W
QW
oral
Ultomiris gMG launch underway, ALXN2050 Phase II FPCD
gMG portfolio
breadth
expands
addressable
patient
population
1
Ultomiris first step to expand reach in gMG
29
3x addressable patient population
1
, new market entrants expand branded market
1. Ultomiris addressable population c.30k patients in the US compared to 8-10k for Soliris, due to requirement to fail two prior IST regimens in Soliris Phase III REGAIN trial. gMG = generalised myasthenia gravis
2017-2021 Present
Branded market
expansion
of Ultomiris
utilization since launch
from complement naïve
patients
Branded
Unbranded and off-label
Ultomiris offers continuous disease control with minimal
doses per year, compared to other branded medicines
1/3
Alexion portfolio approach in IgAN and LN
30
Complement inhibition in renal represents potential multi-blockbuster opportunity
1. Renal class III-IV, with or without class V. IgAN = IgA nephropathy; LN = lupus nephritis; PoC = proof-of-concept; EU5 = France, Germany, Italy, Spain, United Kingdom; FPCD = first patient commenced dosing.
PoC trials with Ultomiris and ALXN2050 in renal indications
Lack of approved treatments,
significant unmet need
Diagnosed
patients
(US, EU5, JP)
Treatment
landscape
IgAN LN
1
>250k >130k
Ultomiris trial achieved 80% enrollment
in IgAN and >1/3 enrollment in LN
ALXN2050 Phase II FPCD in IgAN
Trial status
PoC data will inform Phase III investment decision for either Ultomiris, ALXN1720 or ALXN2050
Evidence for the role of alternative and
terminal pathways in IgAN
• Increased levels of C3 proteolytic
fragments associated with
IgAN disease progression
• Urinary C5b-9 elevated in patients
with IgAN
• In-human PoC recently presented
with terminal pathway inhibition
Innovating in new complement frontiers
31
Multiple novel complement platforms, both established and emerging
PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic syndrome; gMG = generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder; GBS = Guillain-Barré syndrome; DM =
dermatomyositis; HSCT-TMA = haematopoietic stem cell transplant-associated thrombotic microangiopathy; CM-TMA = complement-mediated thrombotic microangiopathy; CSA-AKI = cardiac surgery-associated acute
kidney injury; PNH-EVH = paroxysmal nocturnal haemoglobinuria with extravascular haemolysis; GA = geographic atrophy; SCD = sickle cell disease; siRNA = small interfering RNA; cAMR = chronic antibody-mediated
rejection; LCM = lifecycle management.
Five platforms serving multiple
complement-mediated diseases
Range of offerings including orals,
biologics and siRNA
LCM portfolio allows for differentiated
pricing strategy
Multiple assets across development
stages reinforce long-term
complement leadership

Beyond
Complement
BONE
DISEASE &
CARDIOMYOPATHY
32
DONNAN LIVING WITH aHUS
Expanding beyond Complement
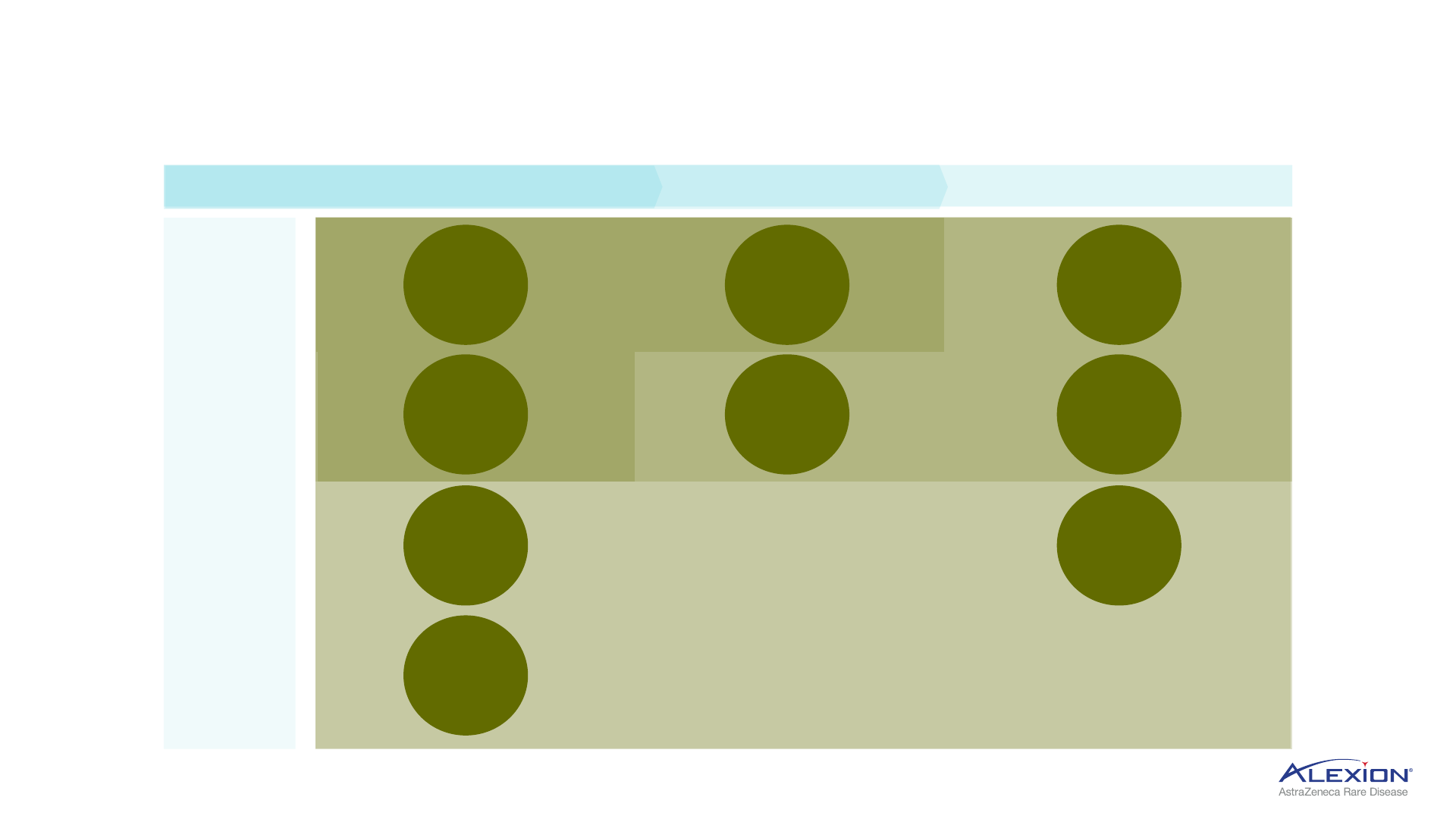
33
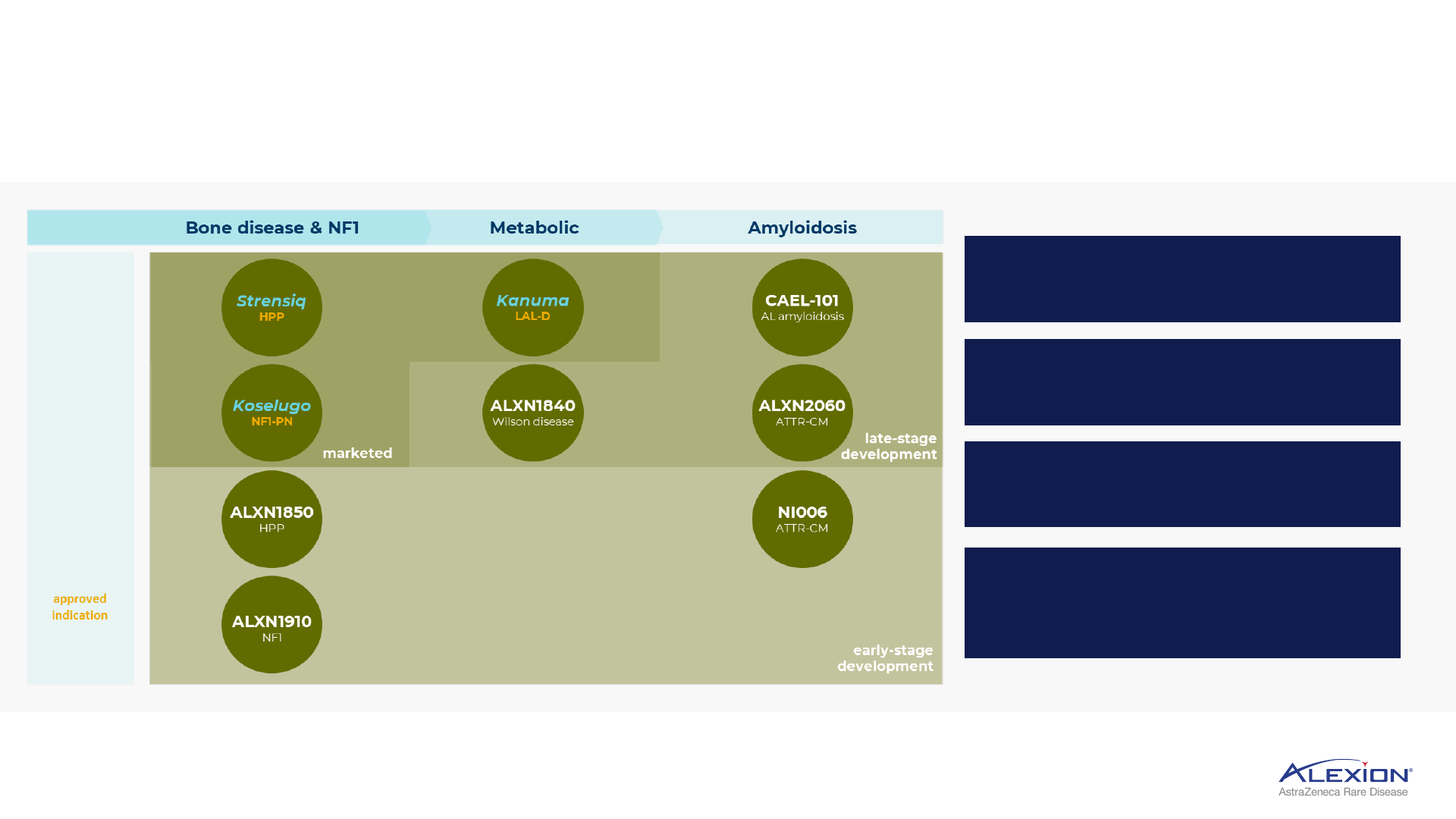
Initial expansion in skeletal manifestations and NF1, metabolic and amyloidosis
HPP = hypophosphatasia; NF1 = neurofibromatosis type-1 with plexiform neurofibromas; LAL-D = lysosomal acid lipase deficiency; AL amyloidosis = light-chain amyloidosis; ATTR-CM = transthyretin amyloid
cardiomyopathy.
Strensiq
HPP
Bone disease & NF1
approved
indication
Koselugo
NF1-PN
ALXN1850
HPP
ALXN1910
NF1
marketed
Kanuma
LAL-D
Metabolic
ALXN1840
Wilson disease
CAEL-101
AL amyloidosis
ALXN2060
ATTR-CM
NI006
ATTR-CM
Amyloidosis
late-stage
development
early-stage
development
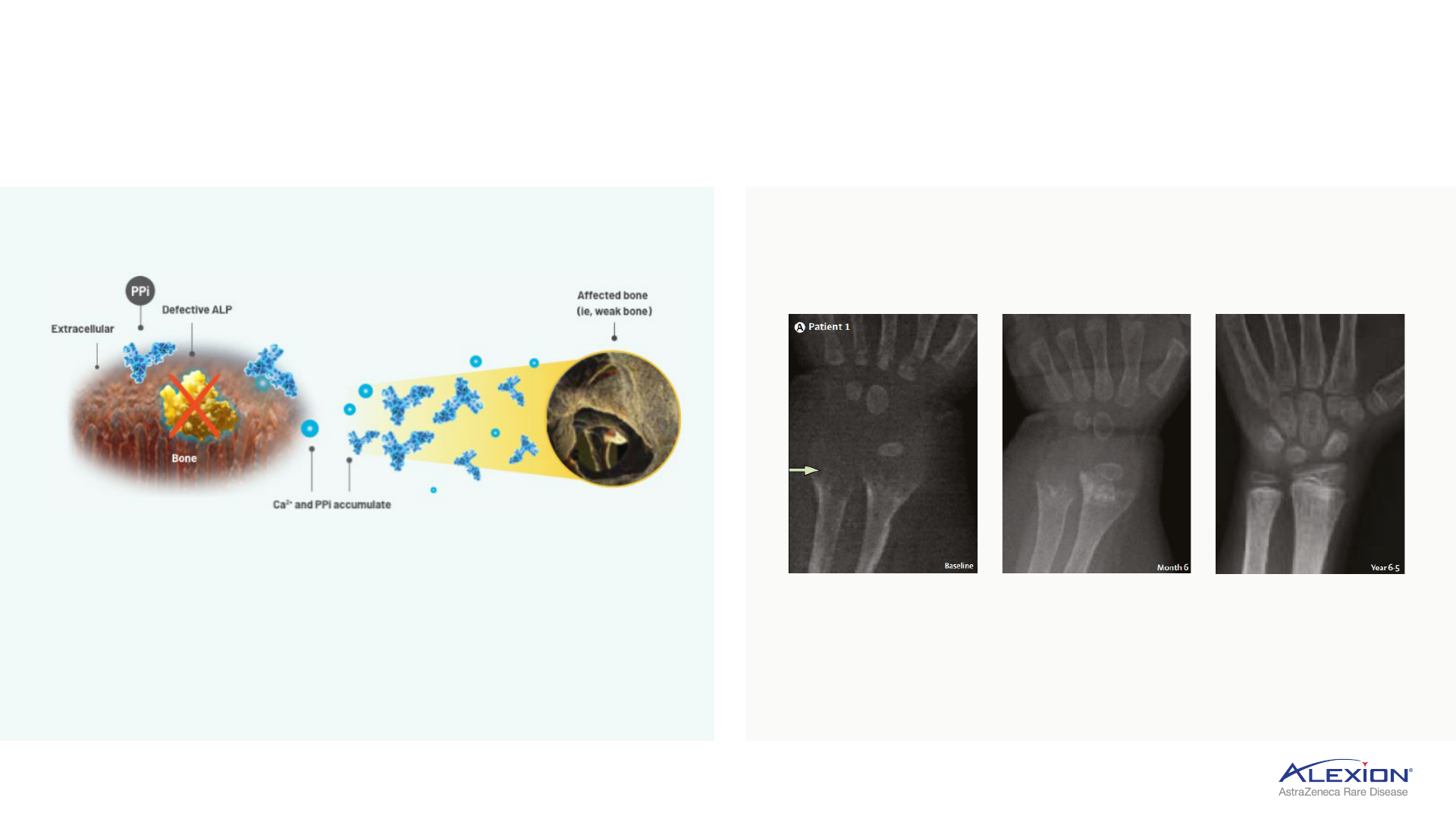
Hypophosphatasia
34
Strensiq is standard of care, foundational ERT for HPP patients
ERT = enzyme replacement therapy; HPP = hypophosphatasia; ALP = alkaline phosphatase; PPi = inorganic pyrophosphate; ALPL = TNSALP production gene; TNSALP = tissue-nonspecific ALP.
• Mutations in ALPL gene cause low ALP activity
• PPi accumulates and prevents bone mineralization,
resulting in skeletal defects and multi-systemic
complications
• HPP is an ultra-rare disease, defined as <6,000 in US
Inherited metabolic disorder characterised by
ALP deficiency
Clinical manifestations of HPP
Radiographic changes from baseline to year 6.5 in patients treated
with Strensiq
Strensiq replaces deficient tissue-nonspecific ALP
(TNSALP) enzyme to enable bone mineralisation

35
ALXN1850: next-generation HPP
Patient-centered innovation, optimised molecule to extend half-life, less frequent dosing
• Longer half-life, less frequent QW
SC dosing
• Improved PK
• Increased enzymatic activity
• Higher bioavailability
• Higher in-vivo exposure
• Improved manufacturing process
ALXN1850
1. Increase in addressable population driven by expanded indication of ALXN1850 to include patients with adult-onset HPP (vs perinatal/infantile and juvenile onset only with Strensiq (ex-JP)) HPP = hypophosphatasia; QW = once-
weekly; SC = subcutaneous; PK = pharmacokinetics.
Planning to initiate Phase III trial in 2023
Strensiq
Perinatal/infantile
and juvenile-onset HPP
ALXN1850
Patients 2 years
and older with HPP
>2x addressable
population
1
36
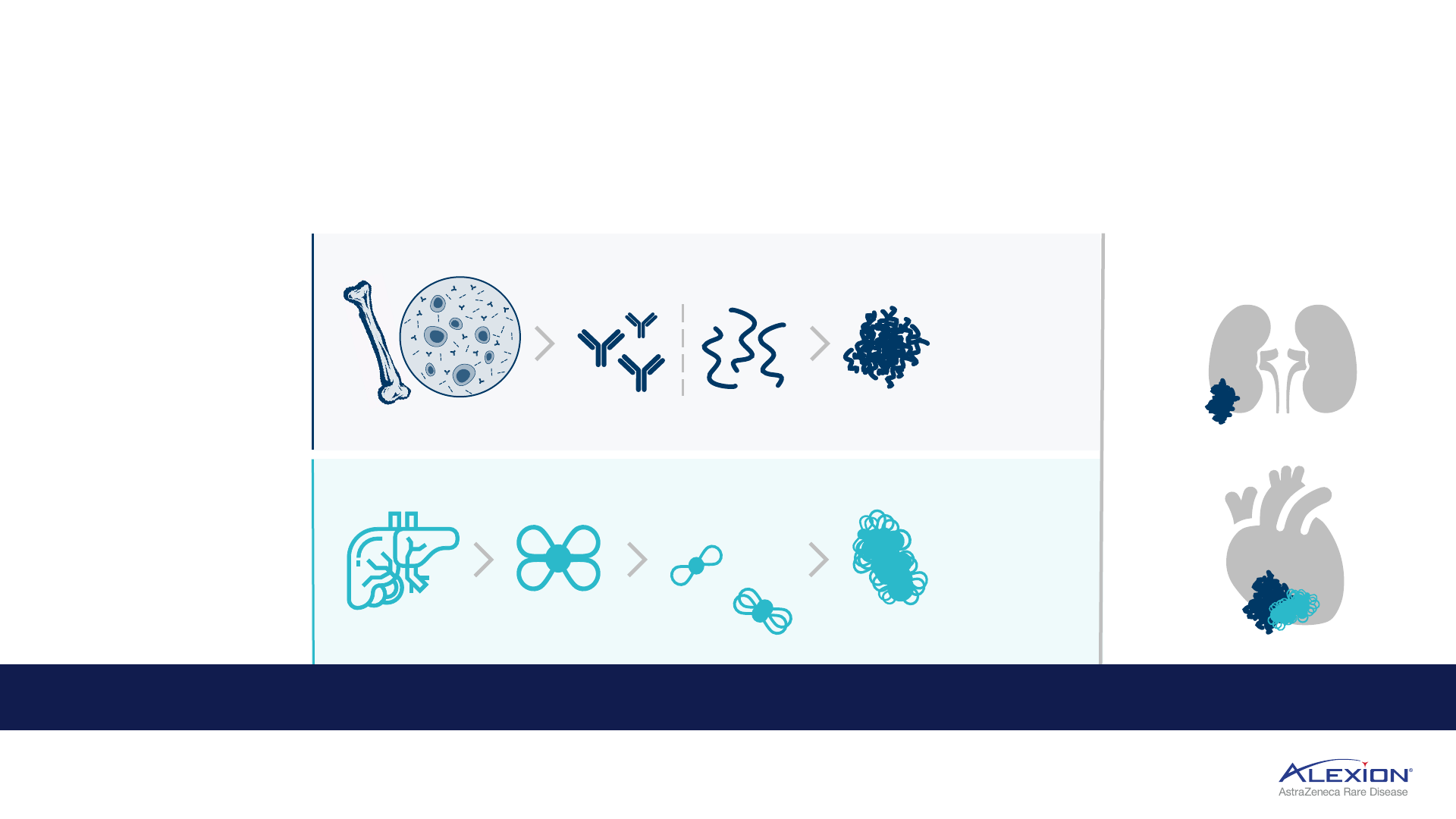
Amyloidosis
Progressive accumulation of toxic amyloid fibrils in tissues and organs
amyloid deposition
primarily impacting:
ATTR amyloidosis:
fatal
disease caused by the deposition
of TTR amyloid fibrils, leading to
cardiomyopathy and
polyneuropathy
• With a cardiopathy, life
expectancy 1-5 years with only
1-2 years NYHA class III-IV
patients
TTR produced in liver
free
tetramers
formed
AL amyloidosis: fatal
disease caused by the deposition
of light chain amyloid fibrils,
leading to multiorgan
dysfunction and failure
• Primarily impacts kidney and
heart in >60% of patients
• Median OS in most severe
stage (IIIb) is 4 months
misfolded pre-amyloid light
chain proteins created by
abnormal plasma cells
collection of
misfolded proteins
form insoluble
amyloid fibrils that
deposit and
accumulate
in ATTR, free tetramers
disassociate to form:
misfolded
monomers
oligomers
collection of
monomers and
oligomers form
amyloid fibrils that
deposit and
accumulate
kidneys and/or heart
Amyloid deposition leads to progressive organ damage or failure that can ultimately be fatal
AL amyloidosis = light-chain amyloidosis; OS = overall survival; ATTR amyloidosis = transthyretin amyloidosis; TTR = transthyretin; NYHA = New York Heart Association.
plasma cells produced
in bone marrow
37
Amyloidosis portfolio strategy
CAEL-101, NI006 novel mAb depleters designed to bind and clear amyloid fibrils
ATTR-CM
NI006
ATTR-CM
Planning to initiate Phase III 2023
274k Top 8
44k US, 39k EU5
Ability to clear toxic fibril deposition in tissues may reverse course of disease
mAB = monoclonal antibody; AL Amyloidosis = light chain amyloidosis, Top 8 = US, EU5, JP, CN; EU5 = France, Germany, Italy, Spain, United Kingdom; AL-CM = light-chain amyloid cardiomyopathy; ATTR-CM =
transthyretin amyloid cardiomyopathy.
CAEL-101
AL-CM
AL amyloidosis
Phase III programme enrollment ongoing
c.20k US, EU5
45k Top 8
First-and-only medicine for both Stage IIIa and IIIb
AL amyloidosis patients
Designed to show overall survival benefit given CAEL-101
targeted MoA to bind and clear amyloid fibrils
CAEL-101 in AL amyloidosis
38
Tailored to address mortality cause by removing amyloid fibrils, improving overall survival
Phase III CAEL-101 twin study
Stage I Stage II Stage IIIa Stage IIIb
# risk factors
1
evaluated
median OS
(months)
0 1 2 2
130 54 – 72 24 4
other assets in development
focused on earlier stages
CAEL-101-302
HLR >2023
CAEL-101-301
HLR >2023
CAEL-101
R 2:1
CAEL-101 + PCD treatment
placebo + PCD treatment
CAEL-101-301
Stage IIIb
(n=124)
R 2:1
CAEL-101 + PCD treatment
placebo + PCD treatment
CAEL-101-302
Stage IIIa
(n=267)
Minimum 12-month active treatment QW IV infusions for
4 weeks, then Q2W
1. Risk factors include cTnT and NT-proBNP. AL amyloidosis = light-chain amyloidosis; PCD = plasma cell dyscrasia; QW = once-weekly; IV = intravenous; Q2W = every 2 weeks; MoA = mechanism of action; OS = overall
survival; HLR = high-level results.
Expanding beyond Complement
39
Initial expansion opportunities in skeletal manifestations, metabolic and amyloidosis
HPP = hypophosphatasia; NF1 = neurofibromatosis type-1 with plexiform neurofibromas; LAL-D = lysosomal acid lipase deficiency; AL amyloidosis = light-chain amyloidosis; ATTR-CM = transthyretin amyloid
cardiomyopathy.
Novel amyloid fibril depleters with
CAEL-101, NI006
Opportunity to treat range of NF1
patients with Koselugo and NF1
patients with skeletal manifestations
with ALXN1910
Strensiq in HPP is $1bn+ franchise
and growing
Opportunity to expand geographic
reach in HPP with ALXN1850

Expansion
GEOGRAPHIC
40
Geographic expansion
41
Ambition to expand direct presence into nearly 100 countries by 2030
Leveraging AstraZeneca’s geographic footprint
to enable rapid expansion, predominantly in EM
1
Emerging Markets represent significant
growth opportunity to 2030
High-teens % CAGR
for EM revenues to 2030
c.25% of international
2
revenue
comes from EM by 2030
%
25
1. Reflects direct market presence. 2. International excludes US and Japan. EM = Emerging Markets; CAGR = compound annual growth rate.
2021
2023
2030
20
countries
>40
countries
c.100
countries
42
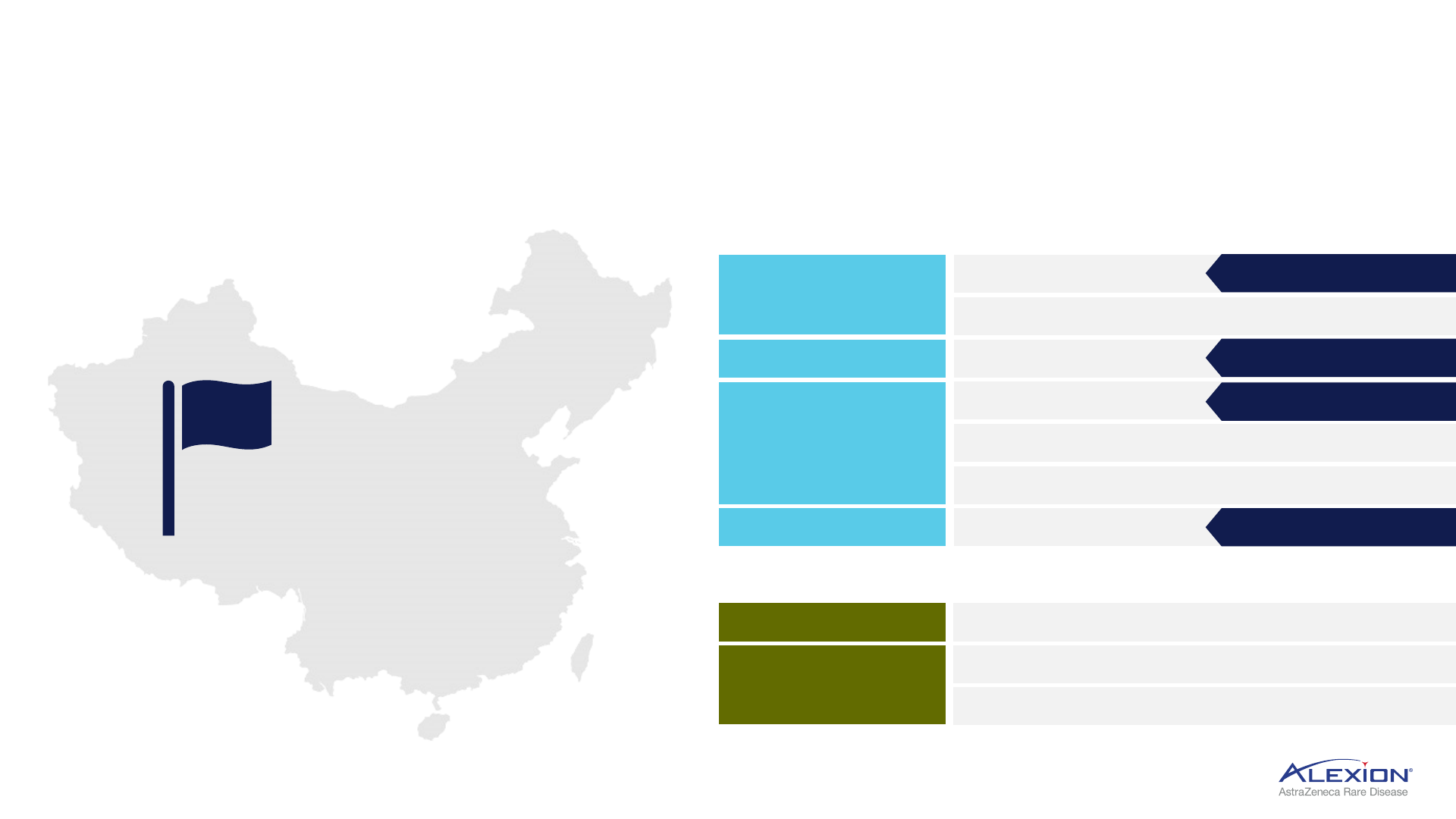
China represents significant opportunity for rare disease
Ambition to launch 10 trials with 10 potential approvals by 2028
1. In final stages of approval. PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic syndrome; gMG = generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder;
HPP = hypophosphatasia.
Established rare disease
unit in China
2021
Complement
Beyond Complement
PNH
Soliris
ALXN2050
aHUS
ALXN2050
ALXN1720
Soliris
NMOSD
gMG
Amyloidosis
CAEL-101
HPP
Strensiq
ALXN1850
2023 Approval
1
Soliris
2023 Approval
1
Soliris
2023 Approval
2023 Approval

43
Scientific
Bridges
ORGANIC
INNOVATION
Accelerating discovery and research
Scientific bridges enable collaboration across Alexion and AstraZeneca
44
V
H
H = single domain antibody.
Advancing
small molecule
discovery
Potential to apply
oral Factor D in
non-rare applications
Genomic
Medicines
Potential to build
genomic medicines
portfolio with existing
AstraZeneca capabilities
Library
exchange
Collaborating to discover
large molecules
(V
H
H library, full length
antibody library,
humanized mouse)
Three genomic medicine projects underway
Leveraging existing AstraZeneca capabilities and applying to rare disease
45
Gene therapy
Antisense
oligonucleotides
Gene editing
• Novel AZN AAV capsids
• In-house promoters
• Innovative ASO-mediated
exon skipping
• AstraZeneca proprietary
CRISPR platform
• Superior safety profile
AZN = AstraZeneca; AAV = adeno-associated virus; ASO = antisense oligonucleotides; CRISPR = clustered regularly interspaced short palindromic repeats.

46
Summary
CLOSING
Alexion, AstraZeneca Rare Disease
1. Based on EvaluatePharma revenue projections for all companies (orphan non-oncology sales); NME = new molecular entity; CAGR = compound annual growth rate.
Supporting AstraZeneca’s industry-leading growth profile, delivering pioneering science
47
Alexion by 2030
>5 NME launches 5-6x patient growth across portfolio
Expand into c.100 countries, Emerging Market
high-teens % revenue CAGR
Leading rare disease company by 2027
1
Sustained
Leadership in
Complement
Expanding Beyond
Complement
Organic Innovation &
Collaboration with
AstraZeneca

48
Marc Dunoyer
Chief Executive Officer,
Alexion
Scott Weintraub
VP, Global Marketing &
Commercial Strategy
Gianluca Pirozzi
SVP, Head of
Development & Safety
Sharon Barr
SVP, Head of Research
& Product Development
Q&A Session
RARE DISEASE
INVESTOR EVENT

Use of AstraZeneca slides from conference calls and webcasts
The AstraZeneca webcast, conference call and presentation slides (together the ‘AstraZeneca materials’) are for your personal, non-commercial
use only. You may not copy, reproduce, republish, post, broadcast, transmit, make available to the public, sell or otherwise reuse or commercialise
the AstraZeneca materials in any way. You may not edit, alter, adapt or add to the AstraZeneca materials in any way, nor combine the AstraZeneca
materials with any other material. You may not download or use the AstraZeneca materials for the purpose of promoting, advertising, endorsing
or implying any connection between you (or any third party) and us, our agents or employees, or any contributors to the AstraZeneca materials.
You may not use the AstraZeneca materials in any way that could bring our name or that of any Affiliate into disrepute or otherwise cause any loss
or damage to us or any Affiliate. AstraZeneca PLC, 1 Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, CB2 0AA. Telephone + 44 20
3749 5000, www.astrazeneca.com
49
